
In the intricate tapestry of scientific discovery and technological advancement, molarity emerges not just as a fundamental concept in chemistry, but as a linchpin in a multitude of groundbreaking research and innovations. From the microscopic intricacies of cell biology to the vast complexities of environmental science, the role of molarity transcends the boundaries of disciplines, proving to be an indispensable tool in deciphering the mysteries of the natural world and shaping the future of scientific endeavors. This article ventures into the captivating realm of molarity, unveiling its pivotal role in recent scientific breakthroughs and its profound implications across various fields.
Molarity, a concept that might seem confined to the pages of chemistry textbooks, is in fact a dynamic force driving numerous scientific breakthroughs and innovations. This article explores the fascinating world of molarity, unveiling its critical role in recent scientific advancements and its far-reaching implications in various fields. Whether you’re a seasoned researcher, an industry professional, or simply a science enthusiast, understanding the latest developments in molarity can offer profound insights into the workings of the natural world.
Decoding Molarity
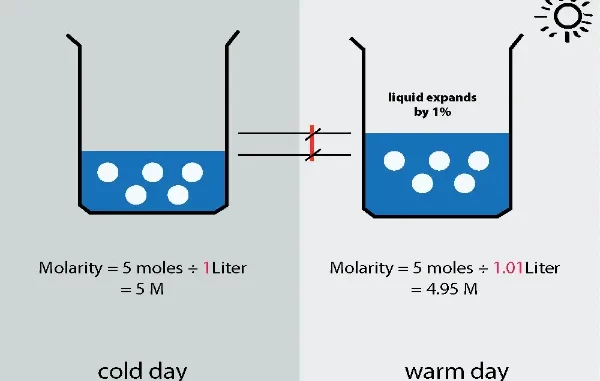
Molarity, denoted as ‘M’, measures the concentration of a solute in a solution, defined as the number of moles of solute per liter of solution. This measure is vital in ensuring precision and consistency across various scientific and industrial processes. For accurate and convenient molarity calculations, tools from Omni Calculator are invaluable.
The Formula at Its Core
The formula for molarity is:
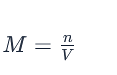
where ‘M’ represents molarity, ‘n’ is the number of moles of solute, and ‘V’ is the volume of the solution in liters. This fundamental calculation underpins many scientific and industrial endeavors.
Recent Scientific Breakthroughs Involving Molarity
Optogenetics in Plant Biology
A groundbreaking study in optogenetics has unveiled a new acid sensor in plant cells. This sensor, interacting with a cell-internal calcium store, could revolutionize our understanding of cellular processes, where molarity plays a critical role in the functionality of cellular components.
Nanophysics and Ion Flow
Researchers from Osaka University have made significant strides in nanophysics by studying thermal energy changes across nanopores that control ion flow. This process, deeply influenced by the concentration of ions or molarity in the solution, could lead to new insights in nanoscale thermal dynamics and material development.
Nanomaterials and Catalyst Efficiency
In the field of nanomaterials, attention has been focused on hollow-structured supported metal catalysts, or nanoreactor catalysts. These catalysts, with their encapsulated active sites and well-defined shells, are ideal for reactions where molarity is a key factor, influencing the efficiency and outcome of these reactions.
Molarity in Drug Development
In drug development, molarity is crucial in determining the effective concentration of pharmaceutical compounds, ensuring their safety and efficacy. It is instrumental in establishing dose-response relationships, a vital aspect of clinical trials.
Environmental Chemistry
Molarity is essential in environmental chemistry for analyzing pollutants in water bodies. Accurate measurement of pollutant concentration is vital for assessing environmental health and formulating regulatory policies.
Food and Beverage Industry
The food and beverage industry relies on molarity to maintain product quality and consistency. Adjusting the concentration of acids and sugars in beverages using molarity calculations ensures taste and safety standards.
Future Prospects of Molarity
Nanotechnology
In nanotechnology, molarity is crucial in synthesizing nanomaterials with specific properties. Precise control over reactant concentration leads to nanoparticles with desired sizes and shapes, essential in various applications.
Green Chemistry
Molarity also finds applications in green chemistry, aiding in developing environmentally friendly chemical processes. Optimizing reactant concentration can minimize waste and enhance reaction efficiency.
Biotechnology
In biotechnology, molarity is used in bioassays and culture media formulation. It ensures optimal growth conditions for microorganisms and cells, crucial in biotechnological product development.
Conclusion
Molarity, far more than a mere academic concept, is a vital player in the ongoing narrative of scientific research and industrial innovation. Its role in ensuring precision and consistency in experiments and processes is invaluable. As science and technology continue to evolve, the applications of molarity are expanding, underscoring its significance in the modern scientific landscape.
For those immersed in scientific research, industry applications, or simply captivated by the marvels of science, grasping and utilizing the principles of molarity is crucial. In this context, resources like Omni Calculator become invaluable. Omni Calculator stands out as a comprehensive platform, offering an extensive array of versatile tools, particularly in the physics and chemistry categories. These tools simplify complex calculations in these fields, making them more accessible and understandable to a broad audience.
Leave a Reply